Proper weed management
Millet has a rather slow early growth and does not rapidly develop a dense canopy cover, which can help to smoother weeds. This makes the crop sensitive to competition by other plants during this time. Weed competition in early growth results in yield loss. Thus weed control prior to planting and until the crop has fully established is important. When the millet plants have produced enough biomass, they compete well with late-emerging weeds, when grown in dense stands.
Weed control options in organic millet production
Conventional farmers rely on the use of herbicides for weed control, when the need arises. Smallholder farmers strive for optimizing non-chemical preventive and direct measures to control weeds. Note: the use of herbicides is not allowed in certified organic agriculture.
Preventive measures
Preventive measures include i) using clean, weed-free seeds, ii) associating millet with crop(s) with good weed suppressing qualities, iii) choosing appropriate spacing, iv) selecting cultivars with good vigour at emergence and strong tillering, and v) applying proper crop rotation. Controlling weeds along ditch banks, roadsides, and field margins will also help prevent weed seeds from infesting the fields.
Mechanical control
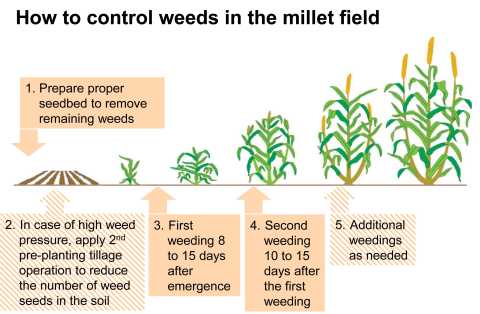
Before sowing millet, any weeds present in the field should be removed through proper seedbed preparation. Crop residues and the removed weeds can be used to cover the soil as mulch or can be aligned at intervals as trash lines in order to cover the soil in between the rows and hinder new weeds from growing. Alternatively, the weeds can be collected and used for making compost, but care must be taken not to disperse weed seeds and diseases through the compost, if the compost is not well prepared. In case of high weed pressure additionally a preplant tillage operation is recommended to kill the weed seedlings prior to planting and to reduce weed pressure in early growth of millet. Light harrowing after emergence can help to control early weeds.
For good cultural practice, two weeding and thinning-out operations are usually necessary in millet. Eight to fi fteen days after emergence (preferably after rain) the field should be thinned to 2 to 4 plants per station. When weeding by hand thinning is usually combined with weeding. The fi rst weeding should be no later than 15 to 20 days after emergence. The second weeding should be done manually and follow the fi rst by 10 to 15 days, but could vary depending on weed pressure. Thereafter, additional weeding should be performed as needed.
Weeding is commonly done with a hoe, but animal-drawn or tractor-drawn cultivators can be used, too. In case a drawn cultivator is used for inter-row cultivation, the weeds between plants within the rows are removed by hand.
Broadcast millet can be mechanically weeded using a tine-weeder (a harrow with spring steel tines). Tine-weeding is very effective between sowing and plant emergence (called blind harrowing), as well as when the millet plants have three to four leaves and the weeds are not taller than 1 cm.
Cultural control
Planned rotation or intercropping of millet with leguminous crops or green manures will prevent the build-up of weeds, including Striga. Striga does not do well in soils with a good fertility status. The combination of different weed management practices will most effectively reduce weed proliferation.
Controlling the Striga weed
Striga hermonthica (witch weed) is a parasitic weed, which is problematic in many cereal producing areas of Africa. Striga competes with the millet plants for both water and nutrients. Consequently low soil fertility and low rainfall favour
Striga infestations. To prevent Striga from spreading, farmers should plant uncontaminated seeds and clean off soil and plant debris from machinery, shoes, clothing, and tools before entering fields. If the Striga plants are few, pulling-out the weed before it produces seed is an option, but not a long-term solution. Another method called trap cropping involves planting a species in an infested field that will induce the Striga seeds to germinate but will not support attachment of the parasite. Example of such crop is plumed cockscomb (Celosia argentea) planted in rotation with millet. The plumed cockscomb will ‘trick’ the Striga seed to germinate, but will not support any further growth of the weed seedlings. This helps to deplete the Striga seed bank in the soil while preventing formation of more seed by the weed. Other crops that are claimed to be effective against Striga include cotton, sunflower, groundnut, castor, dolichos bean, and linseed. In the push-pull system Desmodium species (silverleaf, D. uncinatum and greenleaf, D. intortum) are used to repell the stemborer moths. The same Desmodium species also control Striga.
Effective pest management
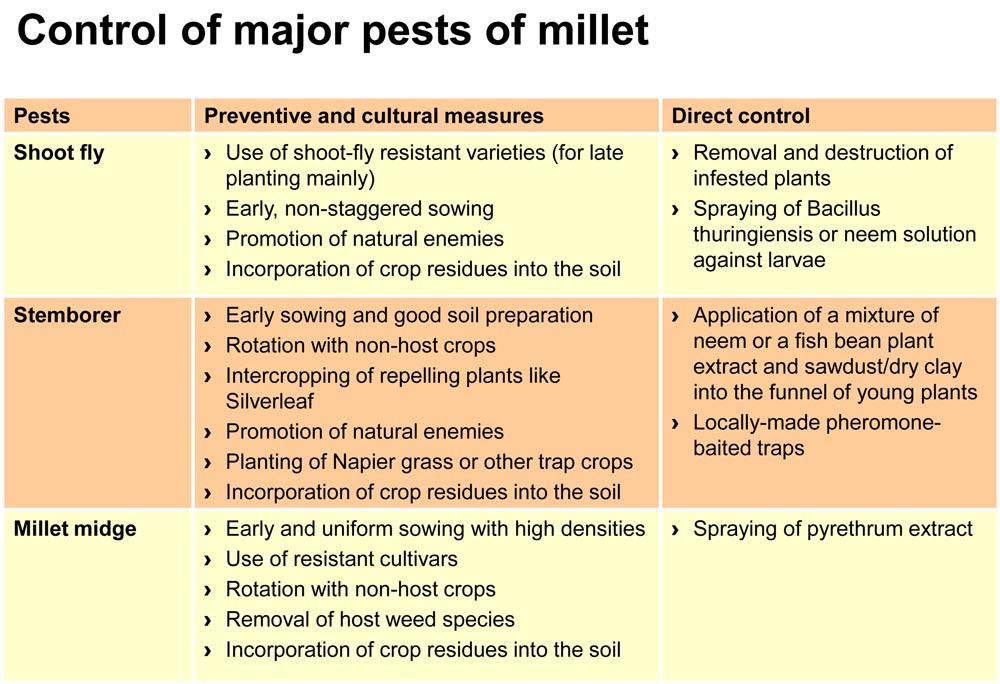
Shoot fly, stemborer, earworm and millet midge are the most important pests in millet, but also grasshoppers, locusts, white grubs and various butterfl ies can attack the crop. Birds are also important millet grain pests.
The sorghum shoot fly (Atherigona soccata) is also known to attack millets. It lays single cigar-shaped eggs on the undersides of leaves of young millet plants between the 1- to 7-leaf stages. The eggs hatch after only a day or two, and the larvae feed on the shoot tip. Feeding activity by the larvae results in wilting and drying of the young plant leaving a dead heart (central leaf develops into a dead heart and comes out easily on pulling). If the dead heart is plucked, it releases a bad odour. If the plant builds side tillers, they may also be attacked. The adult shoot flies resemble small houseflies.
Infestations are especially high when millet planting is staggered due to erratic rainfall. Temperatures above 35 °C and below 18 °C reduce shoot fly survival, as does continuous rainfall. Parasitic wasps and several species of spiders are important predators on eggs. Therefore, these natural enemies should be encouraged through maintaining strips with flowering plants around the fields. To reduce carry-over from one season to the other the crop residues should be collected and destroyed after harvest and other sources of mulch should be used in place of the millet or sorghum residues. If available, shoot-fly resistant varieties should be used.
Stemborer (Coniesta igenfusalis) is an important pest of millet, especially of pearl millet. The larvae feed on the growing points, leaves and stems of the plants at different growth stages, resulting in dead hearts. Destruction of crop residues through incorporation into the soil and good soil preparation contribute to control of the stemborer. Proper crop rotation breaks the pest’s life cycle.
Mixed cropping of millet with other species also confuses the pest. Promotion of natural enemies with strips of insect-feeding flowering plants is also helpful, as several natural enemies attack this pest at different stages of its cycle.
The push-pull method, developed in East Africa, is very effective in controlling of cereal stemborer. This involves use of trap crops to attract stemborer colonization away from the millet plants (pull) and intercrops to repel the pests (push) as it is practiced for other cereals. Examples of trap crops are Napier grass, Sudan grass and molasses grass (Melinis minutifl ora). They are planted in three rows around the millet fields. Whereas the fodder legume silverleaf, Desmodium uncinatum, which acts as repellent, is intercropped between the rows of millet.
Direct control is possible with application of neem during the evening.
Also inexpensive, locally-made pheromone-baited traps can be efficient in controlling stemborers. Placing simple locally made pheromone-baited traps along fences, or in the field, disrupts the mating activity of the stemborer moths by almost 90 % and results in a reduction of the population. The pheromone baited traps can also be used as a method for monitoring the stemborer adults to facilitate decision making on control of the larval stages. When the traps are placed in the field, 1,2 meters seems to be a good height at which the traps should be placed above the ground. Spacing between the traps in the field should be about 15 meters. Local extension agents can provide information on where and when to install the traps and where to obtain the pheromones.
Millet midge (Geiromiya penniseti) is abundant during the rainy season. The larvae of the fly feed on the developing seeds. As a result, infested grains do not develop and panicles have a blasted appearance. Appropriate rotation with nonhost crops and intercropping reduce pest damage. After harvest crop residues should be destroyed to destroy the pest (although these would otherwise have been retained in the fields on the soil surface to protect the soil). Ideally fields are ploughed after harvest and shortly before sowing. Spraying of natural pyrethrum is possible, though not very economic.
Birds are the major pests of millet, especially Quelea spp. They prefer millet grains because of their small size. Preventive measures against bird attack in-clude using cultivars with long, hard bristles. Risk of damage can be reduced by planting pearl millet away from tree lines or woods. Scaring of birds for several weeks before the harvest with efficient bird scaring methods is essential.
The lesser grain borer (Rhyzopertha dominica) and the khapra beetle (Trogoderma granarium) attack pearl millet at storage and can cause serious damage.
Rats can also destroy harvested grains. For more information on control of storage pests see under paragraph 12 «Post-harvest handling».
Effective disease management
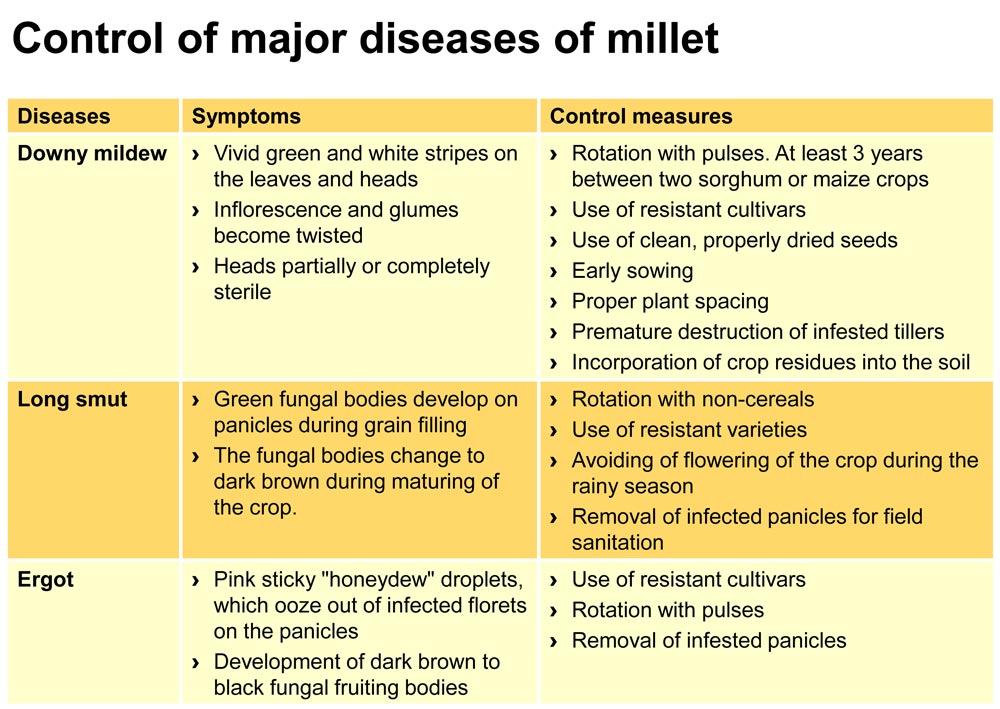
Downy mildew, smut, rust and ergot are widespread diseases where millet is grown in Africa. In general, effective disease management starts with prevention of attack by ensuring clean planting materials or resistant varieties which should be planted in a clean environment followed by proper field sanitary procedures and good husbandry practices. Normally all cultivation practices that encourage plant vigour will enhance the crop’s ability to reduce the impact of disease attacks.
Downy mildew (Sclerospora graminicola) is the most devastating disease in millet and is important in most parts of Africa. The disease is transmitted through the soil, crop residues, contaminated seeds and tools, and is prevalent during rainy periods. Symptoms often vary. Leaf symptoms begin as chlorosis (yellowing between the veins) on the bottom leaves. White fungus may be observed on the underside of infected leaves. Severely infected plants are generally stunted and do not produce panicles. Infl orescence and glumes can become twisted and transform into leafy structures (green ear symptom). Spreading of the disease can be reduced by destroying prematurely infested tillers and infested crop residues.
Some varieties that are resistant to downy mildew have been selected and can be planted, if the risk of downy mildew is high in a particular area. Early sowing is useful also, but is not always feasible due to competition for labour with other crops and the sowing window is often short in rain-fed situations.
Long smut (Tolyposporium penicillariae) attacks the millet plants during flowering by wind-borne spores and rain. Infections are most important when humidity of the air and temperature are high. Green fungal bodies larger than the seed develop on panicles during grain fi lling. As the crop matures, the fungal bodies change in colour from green to dark brown, containing dark spores. Con-trol of the disease focuses on preventive measures such as the use of tolerant or resistant varieties, timing of planting to avoid flowering during the rainy season, and by applying cultural measures that contribute to crop hygiene. With rainfall variability within and across seasons, timing of planting may be diffi cult; however it is important that farmers ensure that they are ready for planting with the fi rst effective rains by having all inputs well before the rainy season starts.
Ergot (Claviceps microcephala) develops after flowering. Pink sticky «honeydew» droplets ooze out of infected fl orets on the panicles. High humidity and temperatures between 20 to 30 °C favour the development of the disease. Within 10 to 15 days, the droplets dry and harden, and dark brown to black fungal fruiting bodies develop in place of seeds. During threshing they generally get mixed with the grain. The disease can be controlled by rotating millet with non-cereals, preferably pulses, growing resistant varieties, and avoiding planting seeds from infected panicles. For good field sanitation affected panicles should be removed and destroyed.



Comments
Post a Comment